22+ What Would Happen To A Cell In An Isotonic Solution Ideas in 2022
What would happen to a cell in an isotonic solution. Although it is technically isotonic while the Dextrose is still in the solution the dextrose gets used up by the bodys cells for energy once it enters the blood stream. In an isotonic solution the flow of water in and out of the cell is happening at the same rate. This is a state of equilibrium and no water moves in or out through the semipermeable membrane. So this remaining solution acts upon the body as if. Animal and plant cell In an isotonic solution Isotonic solution is a solution in which the concentration of solutes is equal so- Water diffuses into and out of the cell at equal rates- Theres no net movement of water across the plasma membrane- The cells retain their normal shape 4. The cell loves to be in an isotonic state and when something happens to make it unequal like with hypotonic or hypertonic conditions it will use osmosis to try to equal it out. What Happens to Water and the Cell in a Hypertonic Solution. If animal and plant cells are kept in isotonic solution then cells will not swell or shrink. An isotonic and hypertonic solution are the. Conversely more solute particles in the ICF causes more water to rush into the cell which may cause it to burst. When cells are in isotonic solution movement of water out of the cell is exactly balanced by movement of water into the cell. Isotonic Solution Definition.
What happens when you place an animal cell in an isotonic solution. A cell is in an isotonic solution if the osmotic pressure inside the cell is equivalent to the osmotic pressure of the solution surrounding the cell. Cells in Isotonic Solutions When two environments are isotonic the total molar concentration of dissolved solutes is the same in both of them. In contrast a hypotonic solution has less solute than inside the cell like putting a cell in distilled water. What would happen to a cell in an isotonic solution An isotonic solution refers to two solutions having the same osmotic pressure across a semipermeable membrane. What Happens to Water and the Cell in a Isotonic Solution They dont gain or lose water through osmosis causing the cell to be equal with no change. A hypertonic solution is a solution that contains more solute than the cell which is placed in it. The morphology of the cell specifically the surface area-to-volume ratio is a critical factor for diffusion of oxygen and carbon dioxide. An isotonic solution is one that has the same osmolarity or solute concentration as another solution. A solution with same osmotic concentration as part of other cell. The solution is isotonic in relation to the cell. A hypotonic solution is a type of solution in which the solute concentration is at a lower concentration in the solution compared to that of the cell. What happens to an animal cell when kept in an isotonic solution.
What would happen to a cell in an isotonic solution Condition of equilibrium amount of water entering into the cell equivalent to.
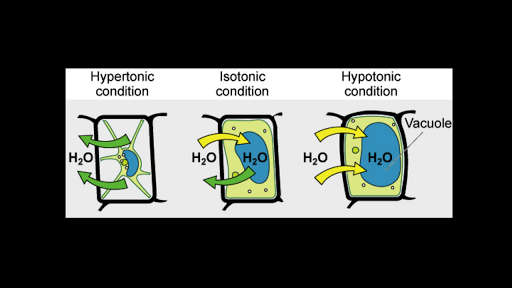
What would happen to a cell in an isotonic solution. An isotonic solution has a solute concentration equal to that inside of the cell. Plant cells have a cell wall surrounding the plasma membrane. Osmosis is the diffusion of water molecules across a semipermeable membrane from an area of lower concentration solution ie higher concentration of water to an area of higher concentration solution ie lower concentration of water.
Isotonic means the concentration is the same as the cells in the potato equilibrium. If animal and plant cells are kept in isotonic solution then cells will not swell or shrink. Each part is made up of a solution and depending on the tonicity of the fluid you can having shifting of fluids from outside of the cell to the inside via osmosis.
The osmolarity of both fluids is equal. Once the dextrose is used up all that is the water or hypotonic saline. Plasma is the primary isotonic solution for red blood cells.
Isotonic hypertonic and hypotonic refers to what happens to materials during passive transport. In this case water will leave the cell since the cell has a lower osmolarity than the extracellular fluid. A 09 solution of NaCl saline is isotonic to animal cells.
If a cell with a NaCl concentration of 09 is placed in a solution of water with a 10 concentration of NaCl the solution is said to be hypertonic. Nothing happens to a plant cell in an isotonic solution. This state allows for the free movement of water across the membrane without changing.
This is because there is no net movement of water between the cell and the solution and thus. If these two solutions are separated by a semipermeable membrane water will flow in equal parts out of each solution and into the otherThe effect is zero water flow between the two solutions although water is moving both ways. Hence there will not be any change in cells.
It could be the movement of water osmosis or other stuff di. An isotonic solution is a solution which contains the same concentration of solute as in a cell. No change occurs to an animal cell when kept in an isotonic solution.
If not stopped the cell will wrinkle and eventually shrivel up and die. As there is equal amounts of free water on either side of the solution and inner cells there is no net gain or. As a result the cell would shrink in what is called plasmolysis.
Hence there will not be any change in cells. An isotonic solution is a solution which contains the same concentration of solute as in a cell. Answer verified by Toppr.
What would happen to a cell in an isotonic solution Answer verified by Toppr.
What would happen to a cell in an isotonic solution. An isotonic solution is a solution which contains the same concentration of solute as in a cell. Hence there will not be any change in cells. As a result the cell would shrink in what is called plasmolysis. As there is equal amounts of free water on either side of the solution and inner cells there is no net gain or. If not stopped the cell will wrinkle and eventually shrivel up and die. No change occurs to an animal cell when kept in an isotonic solution. An isotonic solution is a solution which contains the same concentration of solute as in a cell. It could be the movement of water osmosis or other stuff di. Hence there will not be any change in cells. If these two solutions are separated by a semipermeable membrane water will flow in equal parts out of each solution and into the otherThe effect is zero water flow between the two solutions although water is moving both ways. This is because there is no net movement of water between the cell and the solution and thus.
This state allows for the free movement of water across the membrane without changing. Nothing happens to a plant cell in an isotonic solution. What would happen to a cell in an isotonic solution If a cell with a NaCl concentration of 09 is placed in a solution of water with a 10 concentration of NaCl the solution is said to be hypertonic. A 09 solution of NaCl saline is isotonic to animal cells. In this case water will leave the cell since the cell has a lower osmolarity than the extracellular fluid. Isotonic hypertonic and hypotonic refers to what happens to materials during passive transport. Plasma is the primary isotonic solution for red blood cells. Once the dextrose is used up all that is the water or hypotonic saline. The osmolarity of both fluids is equal. Each part is made up of a solution and depending on the tonicity of the fluid you can having shifting of fluids from outside of the cell to the inside via osmosis. If animal and plant cells are kept in isotonic solution then cells will not swell or shrink.
Indeed lately has been sought by consumers around us, maybe one of you personally. People are now accustomed to using the net in gadgets to see video and image information for inspiration, and according to the name of the post I will discuss about What Would Happen To A Cell In An Isotonic Solution.
Isotonic means the concentration is the same as the cells in the potato equilibrium. Osmosis is the diffusion of water molecules across a semipermeable membrane from an area of lower concentration solution ie higher concentration of water to an area of higher concentration solution ie lower concentration of water. Plant cells have a cell wall surrounding the plasma membrane. An isotonic solution has a solute concentration equal to that inside of the cell. What would happen to a cell in an isotonic solution .
What would happen to a cell in an isotonic solution
What would happen to a cell in an isotonic solution. Hence there will not be any change in cells. An isotonic solution is a solution which contains the same concentration of solute as in a cell. Answer verified by Toppr. Hence there will not be any change in cells. An isotonic solution is a solution which contains the same concentration of solute as in a cell. Answer verified by Toppr.
If you re searching for What Would Happen To A Cell In An Isotonic Solution you've arrived at the right place. We have 51 graphics about what would happen to a cell in an isotonic solution adding images, photos, photographs, backgrounds, and more. In these page, we also provide number of images out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, translucent, etc.
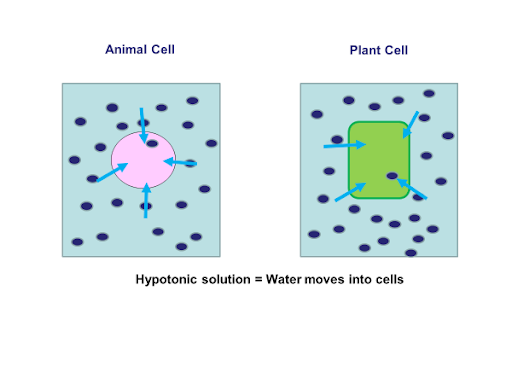
Belum ada Komentar untuk "22+ What Would Happen To A Cell In An Isotonic Solution Ideas in 2022"
Posting Komentar